This post is from an earlier year, meaning the information here is likely outdated. You should look for a newer post from the current year to get the newest exercises and notes.
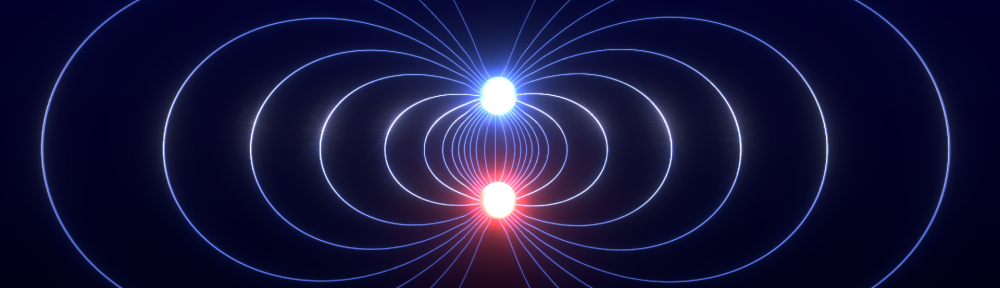
The dipole moment of a configuration of charges is an important concept in electromagnetism, but in most introductory texts it might seem like the dipole moment is only defined for two opposite point charges separated by a distance. This is however not the case. The dipole moment can be defined for any configuration of charges where the net charge is zero.
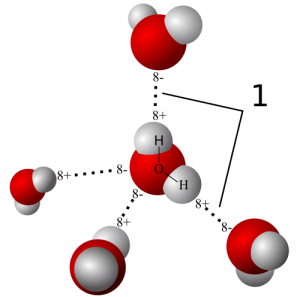
H20 is a polar molecule which means that it has dipole moment. However the H20 molecule is not just two point charges separated by a distance. So how do we then define its dipole moment? Source: Qwerter/Wikipedia
The dipole moment is also crucial in order to undersant how molecules and atoms are affected when they are placed in electric fields as well as understanding the phenomenon of “polarization” of matter.
If you want to learn more about why dipole moments are important and about its generality the following note might be of interest to you: